Slow down the pandemic. The presence of IgG andor IgM antibody to SARS-CoV-2 indicates that the patient has developed an immune response to the SARS-CoV-2 virus.

Dynamics Of Anti Sars Cov 2 Igm And Igg Antibodies Among Covid 19 Patients Journal Of Infection
SARS-CoV-2-specific IgG antibody levels were quantified using two clinically validated and widely used commercial serological assays Architect Abbott Laboratories and iFlash 1800 YHLO detecting antibodies against the spike and nucleocapsid proteins.

What is anti sars cov 2 igg test. However a negative test result does not rule out SARS-CoV-2 virus infection. Elecsys Anti-SARS-CoV-2 is an immunoassay for the in vitro qualitative detection of antibodies including IgG to SARS-CoV-2 in human serum and plasma. Synchronous seroconversion of IgG and IgM means SARS-CoV-2 is not a new virus for the patient because the IgG response needs about two weeks in response to new antigens.
Antibody test SARS-CoV-2 IgA IgG IgM Antibody tests SERION ELISA agile SARS-CoV-2 To be successful in the fight against SARS-CoV-2 it is essential to closely monitor the spread of the virus to enable separation of acutely infected from healthy non-infected persons. This positive result indicates that an individual has developed an immune response to a SARS-CoV-2 infection or a SARS-CoV-2 spike vaccine within the limits of the assay. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine.
To know how many asymptomatic cases have occurred in a population. SARS-CoV-2 Antibody IgG Spike Protein Test Methodology. The Abbott Architect SARS-CoV-2 IgG II assay run under an emergency use authorization from the FDA is quantitative test designed to detect IgG antibodies to the spike protein of SARS-CoV-2 in serum and plasma from individuals with an adaptive immune response to SARS-CoV-2 indicating recent or prior infection.
Antibody tests are as worthless in testing a Covid patient as they are in screening for covid disease Fist of all Cov19 is an infection which starts with the viral infectionWhen a virus infects the virus takes over the hosts biology to create others viruses. In addition to aiding in the diagnosis it also reveals evidence of the previous infection and is thus useful. 2000 High - Out of Range.
SARS-CoV-2 Antibody IgG Spike Protein Date. Binding antibody detection. Qualitative and semi-quantitative chemiluminescent immunoassay CLIA for detection of IgG.
SARS-CoV-2 Antibody IgG Spike Semi-Quantitative test cost max is in RequestATest COVID-19 Coronavirus IgG Antibody Spike Semi-Quantitative Blood Test with price 14900. Both IgM and IgG may be detected around the same time after infection. It is known that not all.
In case of a positive result anti-SARS-CoV-2-IgA was determined additionally5 of 415 asymptomatic outpatients had positive SARS-CoV-2-IgG-antibodies with a calculated prevalence of 12. PKI announced today that the US. June 9 2021 Effective Date.
Results Forty-seven patients mean age 49 years 38 female were included. Reference range of anti-SARS-CoV-2-IgA and IgG was defined as ratio for. June 16 2021 Test Name.
Access SARS-CoV-2 test throughput on Beckman Coulter immunoassay and integrated systems leads the industry at up to 200 tests per hour depending on the analyzer used up to 4800 testsday QC procedures only need to be run once every 24 hours or as required by individual laboratory procedure and the assay comes in 200 testskit requiring less frequent ordering. By continuing to use this site you consent to the use of cookies on your device as described in our cookie policy unless you have disabled themI got a lab test result for COVID-19 Antibody Test SARS-CoV-2 AB IgG Semi-Quantitative. Current sars-cov-2 antibody tests detect igm or igg to viral spike or nucleocapsid proteins.
This laboratory test is available in 4 online lab test stores. The sensitivities of IgG and IgM ranged from 061 27. SARS-CoV-2 Spike Protein IgG Antibody Test The SARS-COV-2 Spike Protein Antibody test is designed to detect the presence and amount of antibodies specific to the SARS-CoV-2 spike protein.
There are two possibilities. The SARS-CoV-2 RBD IgG test is an Enzyme-Linked Immunosorbent Assay ELISA intended for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum. 11 antibody tests may help identify past sars-cov-2 infection if performed two to four weeks after.
The assay uses a recombinant protein representing the nucleocapsid N antigen in a double-antigen sandwich assay format which favors detection of high affinity antibodies against SARS-CoV-2. The SARS-CoV-2 RBD IgG test is. A negative or non-reactive test result indicates that anti-SARS-CoV-2 IgM IgA IgG or all 3 were not detected in the specimen of tested individual.
2021 Jun 2211 71135. SARS-CoV-2 IgGIgM antibody testing is used for rapid screening of SARSCoV2 carriers symptomatic or asymptomatic in hospitals clinics and test laboratories. These tests use purified proteins of SARS-CoV-2 not live virus and can be performed in lower biosafety level laboratories eg BSL-2.
We use cookies to give you the best possible experience on our website. Answer 1 of 2. Serological testing of antiSARSCoV2 IgGIgM has been widely used to diagnose SARSCoV2 infection.
Food and Drug Administration has provided Emergency Use Authorization EUA for its Anti-SARS-CoV-2 S1 Curve TM ELISA IgG. SARS or another coronaviruses surface antigens are the same as SARS-CoV-2 and so the antibody response is the same. The SARS-COV-2 Spike protein IgG antibody test is designed to find out the protective immunity level achieved post vaccination.
The rapid test for SARS-CoV-2 diagnosis provides qualitative detection of IgG andor IgM from human serum whole blood or plasma in approximately 10-15 minutes. Results are reported as AUmL. Assay enabling the measurement of IgG antibodies could yield future discoveries and insights on immune responses to SARS-CoV-2 WALTHAM MassBUSINESS WIREEUROIMMUN a PerkinElmer Inc.
A SARS-CoV-2 semi-quantitative IgG test result is reported as positive at an index3 of 100. COVID-19 Corona Virus Disease is an infectious disease caused by the most recently discovered coronavirus SARS-CoV-2 2019-nCoV. However the diagnostic efficacy of the serum antibody test reported in the earlier studies confused the clinician.
With specific reagents individual antibody types like IgG IgM and IgA can be determined. The ADVIA Centaur SARS-CoV-2 IgG COV2G assay is a chemiluminescent immunoassay intended for qualitative and semi-quantitative detection of IgG antibodies to SARS-CoV-2.

Re Evaluating Positive Serum Samples For Sars Cov 2 Specific Iga And Igg Antibodies Using An In House Serological Assay Clinical Microbiology And Infection
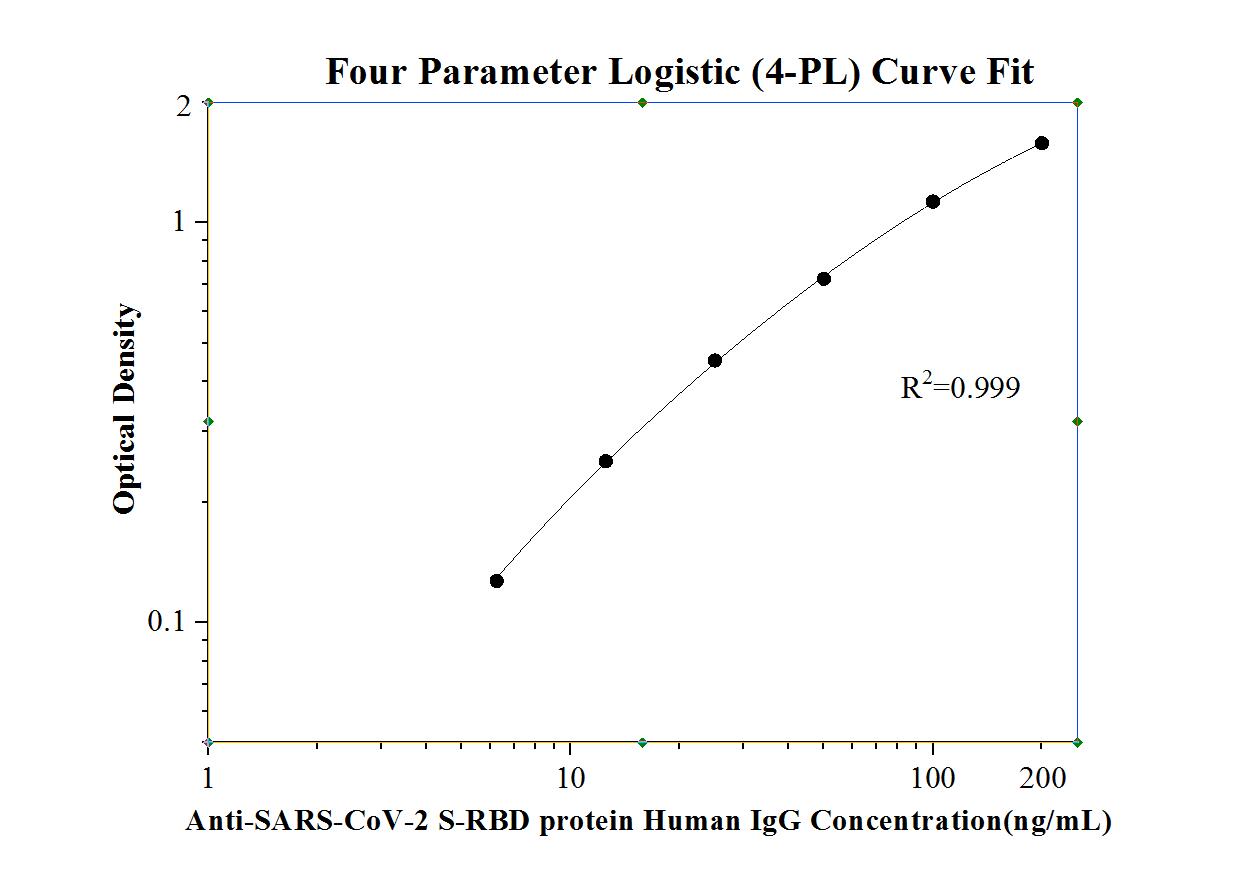
Anti Sars Cov 2 S Rbd Protein Human Igg Elisa Kit Elisa Kit Ke30003 Proteintech
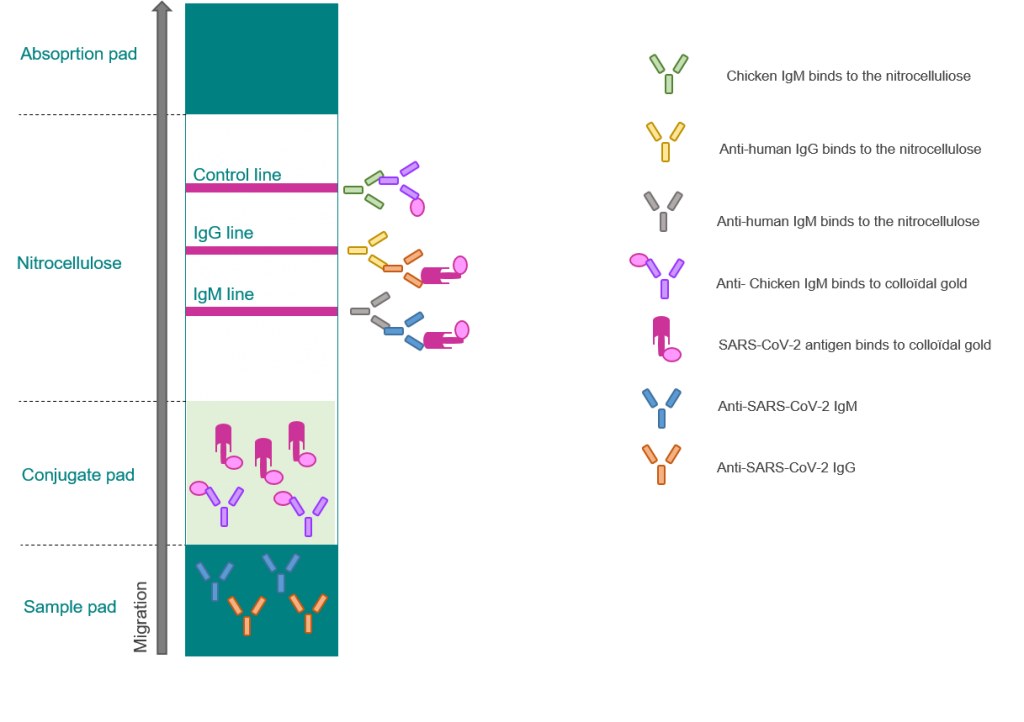
Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
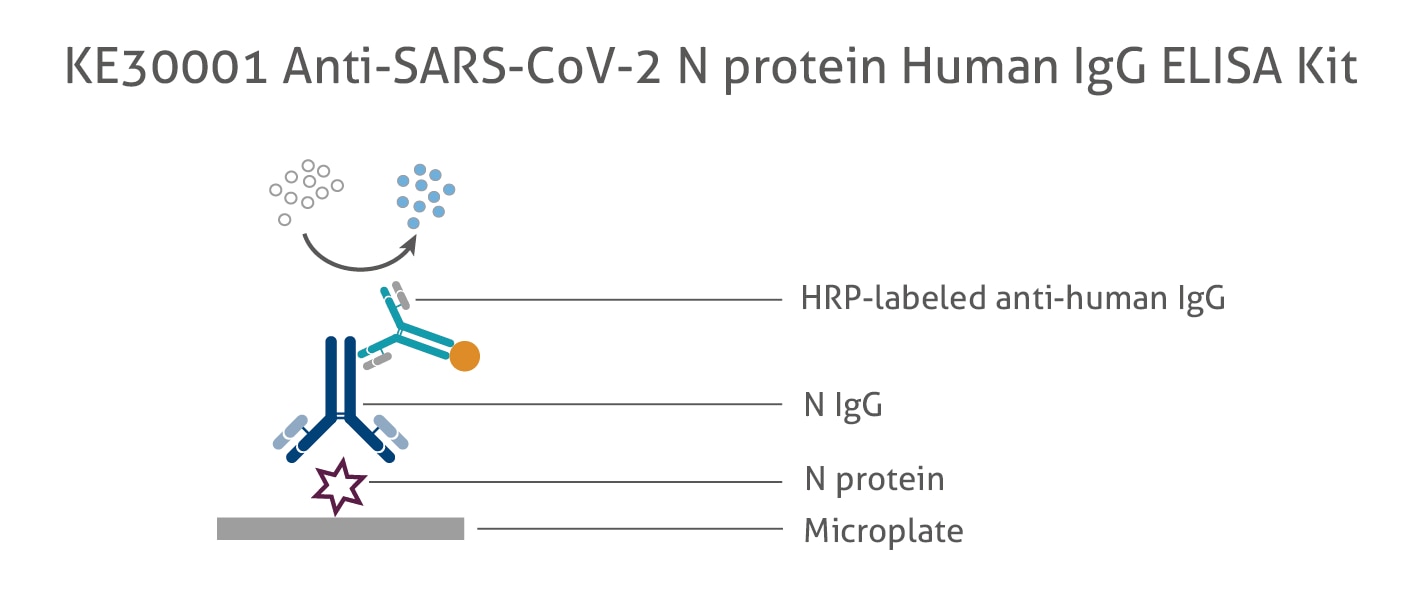
Anti Sars Cov 2 N Protein Human Igg Elisa Kit Elisa Kit Ke30001 Proteintech