This laboratory test is available in 4 online lab test stores. The test targets antibodies against the spike protein.
2000 High - Out of Range.

What is sars cov 2 spike antibody test. The motive of this test is to aware the people whether they have the antibodies that will fight against the spike protein. June 9 2021 Effective Date. The antibody reacts specific to Spike Protein surface glycoprotein RBD domain of Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 COVID-19 100.
The three assays are sensitive and specific for SARS-CoV-2 antibodies. Online Inquiry Add to Basket. Bring this labeled antibody directly to your bench.
What are typical numbers. SARS-Cov-2 Spike RBD protein binding to ACE-2-transfected Human Cell Line is Blocked by SARS-Cov-2 Spike RBD Antibody In a functional flow cytometry test Recombinant SARS-Cov-2 Spike RBD His-tagged protein 10500-CV binds to HEK293 human embryonic kidney cell line transfected with recombinant human ACE-2 and eGFP. Anti-SARS-CoV-2 Spike Monoclonal antibody.
Mouse Anti-SARS-CoV-2 Spike Monoclonal antibody for ELISA IHC-P FCM ICCIF Neut. The results of this semi-quantitative test should not be interpreted as an indication of degree of immunity or protection from reinfection. Current literature suggests that detectable IgG-class antibodies against SARS-CoV-2 develop approximately 8 to 11 days following onset of symptoms.
The SARS-COV-2 Spike protein IgG antibody test is designed to find out the protective immunity level achieved post vaccination. By continuing to use this site you consent to the use of cookies on your device as described in our cookie policy unless you have disabled themI got a lab test result for COVID-19 Antibody Test SARS-CoV-2 AB IgG Semi-Quantitative. Nucleocapsid and spike antibodies were detectable for up to 200 days post-positive SARS-CoV-2 PCR but demonstrated markedly different trends in signal intensity.
The incubation period for COVID-19 ranges from 5 to 7 days. Is 1371 8 standard range in a similar range of people who have been fully vaccinated. You can regularly check the antibody levels in your body to know your immunity status.
SARS-CoV-2 Antibody IgG Spike Protein Date. SARS-CoV-2 Antibody IgG Spike Protein Test Methodology. Qualitative and semi-quantitative chemiluminescent immunoassay CLIA for detection of IgG.
This test is recommended in individuals at least 10 days post symptom onset or following exposure to individuals with confirmed COVID-19. As mentioned previously quantitative determination of anti-SARS-CoV-2 antibodies may help facilitate longitudinal monitoring of the antibody response in individual patients and specifically monitor antibody. The new Elecsys Anti-SARS-CoV-2 S test can quantitatively measure the level of antibodies against SARS-CoV-2 in patients who have been exposed to the virus.
Researchers from the University of Arizona observed in a study published at medRxiv on August 16 that antibodies against the SARSCoV-2 spike protein appeared to wane less rapidly than antibodies against the nucleocapsid protein and persisted for the duration of the follow-up period indicating that humoral immunity is durable for at least several months after SARSCoV2 infection. We use cookies to give you the best possible experience on our website. COVSQ SARS-CoV-2 Spike Ab Semi-Quant S Fees and Codes Fees Authorized users can sign in to Test Prices for detailed fee information.
The antibody was Protein A purified from rabbit antiserum by affinity-chromatography. SARS-CoV-2 antibodies particularly IgG antibodies. The SARS-CoV-2 RBD IgG test uses a recombinant form of the RBD region of the spike protein from SARS-CoV-2 attached to a solid support ELISA plate to capture IgG antibodies that may be present.
Labcorp test details for SARS-CoV-2 Semi-Quantitative Total Antibody Spike This test should not be used to diagnose or exclude acute SARS-CoV-2 infection. Does anyone know the range for results of a COVID-19 Spike Antibody Test SARS-CoV-2 AB IgG Semi-Quantitative. June 16 2021 Test Name.
My doctor is out of town until next week so I cant ask her until then. Others have also reported on interindividual difference in SARS-CoV-2 antibody responses 4 13 yet this is the first report of SARS-CoV-2 antibody trends using a quantitative assay. A positive antibody test can help support a diagnosis when patients present with complications of COVID-19 illness such as multisystem inflammatory syndrome and other post-acute sequelae of COVID-19.
COVIPROTECT Test gives you exact measurement of the antibody titres against SARS CoV-2 virus available in your body against Spike Protein either post vaccination or post infection of COVID 19. Viral tests can also be used as screening tests to reduce the transmission of SARS-CoV-2 by identifying infected persons who need to isolate from others. Clients without access to Test Prices can contact Customer Service 24 hours a day seven days a week.
Current SARS-CoV-2 antibody tests detect IgM or IgG to viral spike or nucleocapsid proteins11 Antibody tests may help identify past SARS-CoV-2 infection if. Lal Clinical Laboratory has started the SARS-COV-2 Spike Protein. The results should always be assessed in conjunction with patients medical history clinical.
SARS-CoV-2 Antibody IgG Spike Semi-Quantitative test cost max is in RequestATest COVID-19 Coronavirus IgG Antibody Spike Semi-Quantitative Blood Test with price 14900. Viral tests including NAATs and antigen tests are used as diagnostic tests to detect infection with SARS-CoV-2 and to inform an individuals medical care. For this purpose Dr.
SARS-CoV-2 Antibody IgG Spike Semi-Quantitative - This test is intended as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2 COVID-19 indicating recent or prior infection. The test says anything greater than 1 is positive but doesnt give the range. SARS-CoV-2 Spike Antibody IgG Description The Abbott Architect SARS-CoV-2 IgG II assay run under an emergency use authorization from the FDA is quantitative test designed to detect IgG antibodies to the spike protein of SARS-CoV-2 in serum and plasma from individuals with an adaptive immune response to SARS-CoV-2 indicating recent or prior infection.
What are typical numbers from a sars-cov-2 semi-quantitative total antibody spike test from labcorp months after vaccination.

Sars Cov 2 Antibody Test From Siemens Healthineers Evaluated Against Other Leading Antibody Assays In Public Health England Study

Sars Cov 2 Antibody Test From Siemens Healthineers Evaluated Against Other Leading Antibody Assays In Public Health England Study
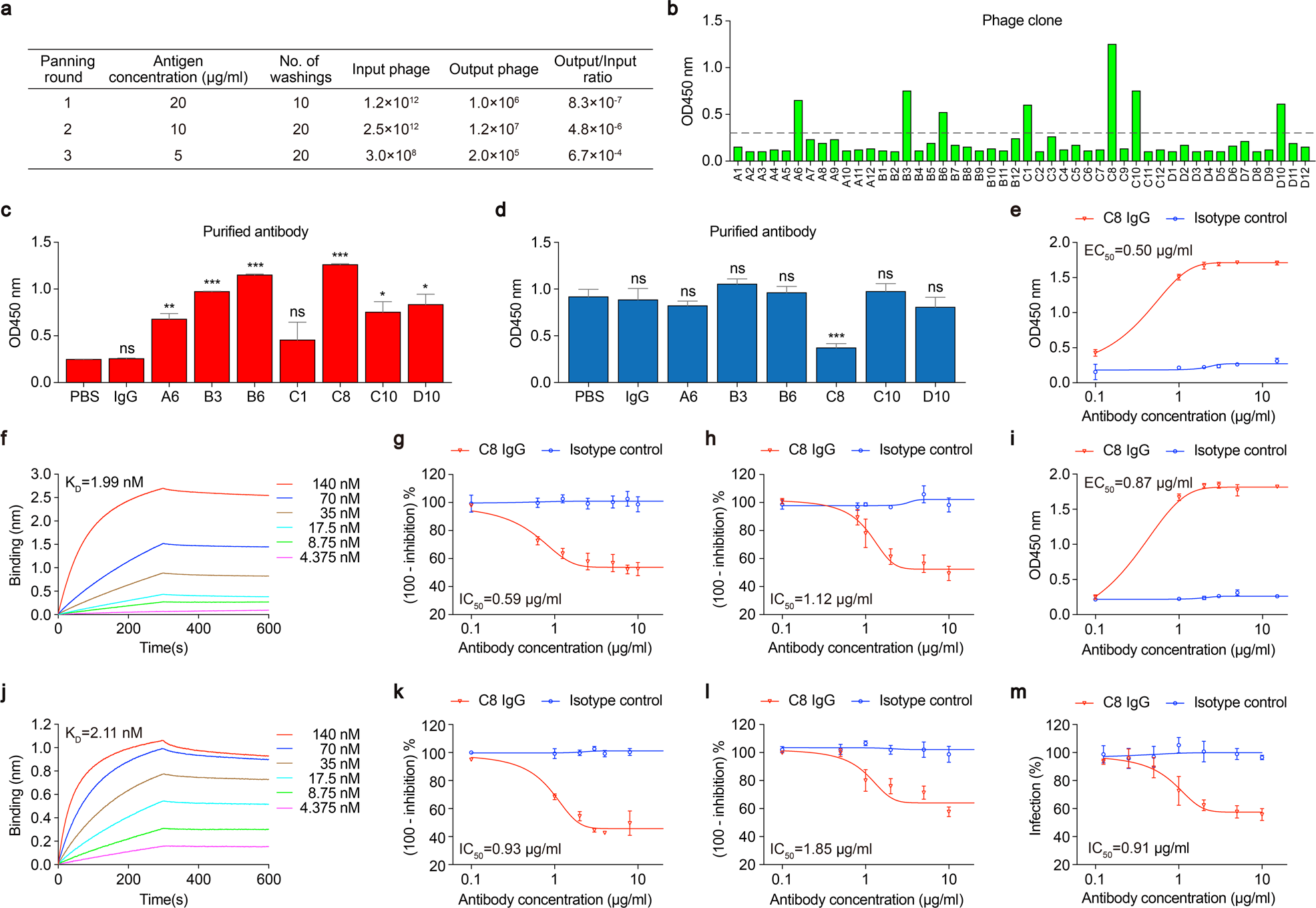
Identification And Characterization Of A Monoclonal Antibody Blocking The Sars Cov 2 Spike Protein Ace2 Interaction Cellular Molecular Immunology
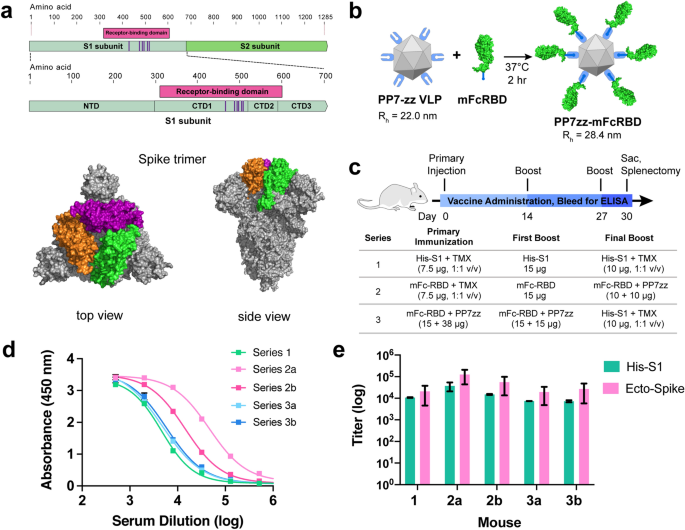
Rapid Development Of Neutralizing And Diagnostic Sars Cov 2 Mouse Monoclonal Antibodies Scientific Reports