The clinical disease is named COVID-19. A PCR test is used to determine if you are currently infected with SARS-CoV-2 COVID-19 which is why many countries are now requesting you provide them with a negative PCR test result before you are allowed entry.

Rapid Detection Of Sars Cov 2 Replicating Or Non Replicating Using Rt Pcr International Journal Of Infectious Diseases
Instructions for Use November 17 2020 Version 20 4 Principle of the Procedure The GenePro SARS-CoV-2 Test is a real-time RT-PCR test.

What is sars cov 2 pcr mean. Akiko Iwasaki1 raises the important question of whether a person with a positive PCR test result for severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 is automatically infectious or infectious only if the cycle threshold Ct is below a certain value eg. A multi-analyte reverse transcription-polymerase chain reaction RT-PCR test such as PKamp Respiratory SARS-CoV-2 RT-PCR Panel 1 permits labs to preserve valuable resources. The UW SARS-CoV-2 Real-time RT-PCR assay targets two distinct regions within the N gene of SARS-CoV-2 the causative agent for COVID-19.
The SARS CoV-2 Lambda Variant. A positive test result means that its likely that you have an infection with SARS-CoV-2. Amplification of both targets results in a presumptive positive detectable test result while amplification of one of two targets results in an inconclusive result and amplification of neither target results a negative non-detectable test result.
What does all this mean for you. 13 th May 2020 Assignment. Means that its likely that you have an infection with SARS-CoV-2.
What do they mean. However from the application point of view the Our findings showed that both tests succeeded in detecting Bayesian modeling is preferable over the conventional statistical SARS-CoV-2 in nasal swab samples RT-PCR and in. March 9 2020 You are being given this Fact Sheet because your samples were tested for the Coronavirus Disease 2019 COVID9 -1 using the Quest Diagnostic SARS-CoV-2.
Results and Follow-Up What do COVID-19 PCR test results mean. The ddPCR assays should be lab validated using our One-Step RT-ddPCR Kit to detect RNA instead of the standard mix for DNA. This is a blood test for antibodies to the virus that causes COVID-19.
SARS-CoV-2 indicates severe acute respiratory syndrome coronavirus 2. B More likely to register a negative than a positive result by PCR of a nasopharyngeal swab. The study analyzed test results from more than 6000 patients and found that using cycle threshold value cutoffs decreased sensitivity significantly.
Such testing should employ either a. You should consult with your health care provider who will monitor your symptoms and provide guidance about how your illness should be managed. Interpreting SARS-CoV-2 RNA Qualitative Real-Time RT-PCR Test Results Updated.
Such assays can alleviate several tests on samples obtained from people who are suspected of having respiratory viral infection which is similar to Covid-19 infection. Heres What You Should Know. SARS-CoV-2 Blood Antibody Test.
NEW YORK A new study published on Monday in the American Association of Clinical Chemistrys journal Clinical Chemistry reinforced AACCs recommendations against using cycle threshold values to determine the performance of SARS-CoV-2 PCR. Severe acute respiratory syndrome coronavirus 2 Colourised transmission electron micrograph of SARS-CoV-2 virions with visible coronae Atomic model of the external structure of the SARS-CoV-2 virion. PCR polymerase chain reaction.
Interpreting SARS-CoV-2 RNA Qualitative Real-Time RT-PCR Test Results Updated. To diagnose a SARS-CoV-2 infection now a nasal swab is used to detect the RNA of SARS-CoV-2 virus. Each ball is an atom.
The Xpert Xpress SARS-CoV-2 test is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in nasopharyngeal swab nasal swab or nasal washaspirate specimen collected from individuals who are suspected of COVID-19 infection. SARS-CoV-2 Variant Assays for ddPCR and RT-PCR These assays were designed specifically for Droplet Digital PCR or RT-PCR using our Expert Design engine which is validated to create high-quality assays. The primer and probe sets are designed to detect RNA from.
Like all RNA viruses SARS-CoV-2 is prone to mutationsadaptive changes that can alter a virus pathogenic potential and other characteristics. Diagnosis of SARS-CoV-2 infection is currently based on real-time reverse transcriptase-polymerase chain reaction RT-PCR performed on either nasopharyngeal swabs NPS or oropharyngeal swabs OPS. This means you have SARS-CoV-2 COVID-19.
September 17 2020 Updated 23 September 2020 Article by. 2 Despite suboptimal detection rates 3 collection of 2. March 9 2020 This Fact Sheet informs you of the significantnown k and potential risks and benefits of the use of the Quest Diagnostics SARS-CoV-2 RNA Qualitative Real.
These mutations can create distinct variants of the virus adding complexity to what has already become the most significant global. Antibodies are produced by the immune system as part of its response to fighting foreign invaders such as viruses. A patient with an OPT result was classified as probable COVID-19 if they met at least one of the.
A PCR test for COVID-19 is a test used to diagnose people infected with SARS-CoV-2 the virus that causes COVID-19. To be clear the virus is named SARS-CoV-2 not COVID-19. Methods All SARS-CoV-2 RT-PCR results performed on respiratory samples with the Roche or the Cepheid tests from 23rd March to 6th August 2020 were collected.
Considerations When Testing SARS-CoV-2 testing may be incorporated as part of a comprehensive approach to reducing transmissionSymptom screening testing and contact tracing are strategies to identify people infected with SARS-CoV-2 so that actions can be taken to slow and stop the spread of the virus. Diagnostic Testing for SARS-CoV-2 Infection Everyone who has symptoms that are consistent with COVID-19 as well as people with known high-risk exposures to SARS-CoV-2 should be tested for SARS-CoV-2 infection. The same question should be asked for other potential sources of viral spread such as inanimate surfaces.
Spike glycoprotein S. To perform the SARS-CoV-2 RNA Qualitative Real-Time RT-PCR test SARS-CoV-2 nucleic acid is first extracted isolated and purified from upper and lower respiratory specimens such as of. Antibodies may appear just a week or so after.
A Detection only occurs if patients are followed up proactively from the time of exposure. This is the COVID-19 PCR test To detect that an infection occurred at some point in the past a serology blood test is done to detect antibodies to SARS.
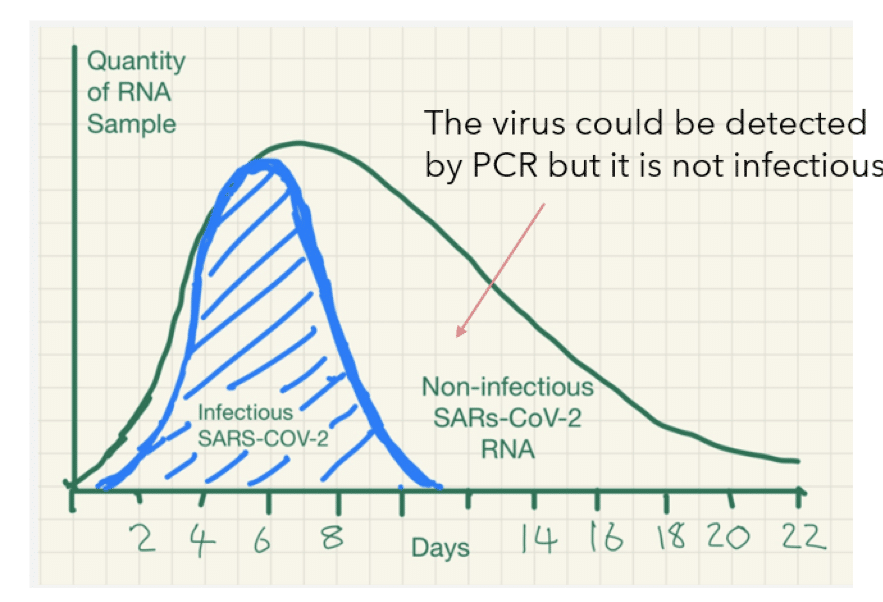
Pcr Positives What Do They Mean The Centre For Evidence Based Medicine
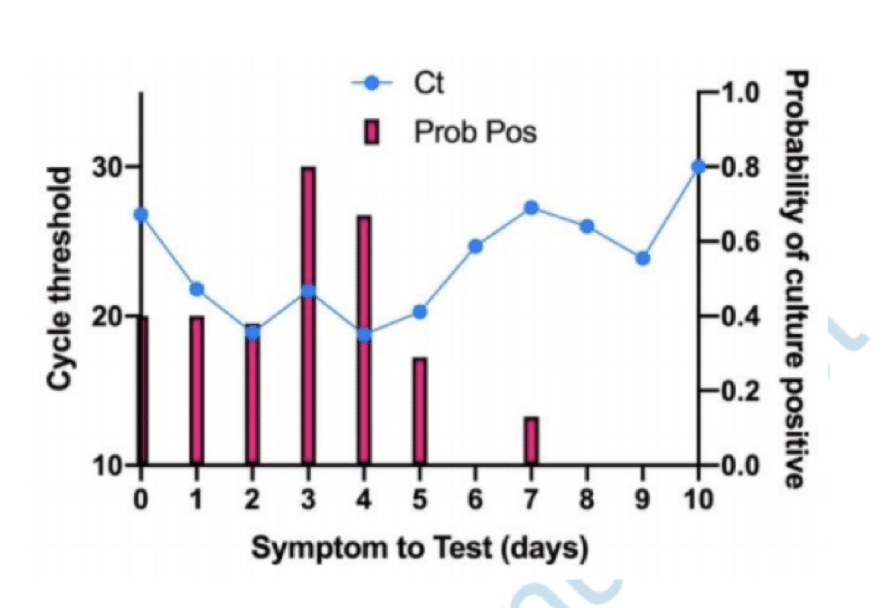
Pcr Positives What Do They Mean The Centre For Evidence Based Medicine

Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine

The Performance Of The Sars Cov 2 Rt Pcr Test As A Tool For Detecting Sars Cov 2 Infection In The Population Journal Of Infection