During the ongoing coronavirus disease 2019 COVID-19 pandemic monitoring patients infected with severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 using viral kinetics or viral loads in various sample types by real-time RT-PCR has become essential. The RT-PCR Test that is done to detect SARS-CoV-2 Virus ie.

Gb Sars Cov 2 Real Time Rt Pcr E N Orf1ab Rdrp General Biologicals Corporation Gbc
The world is facing an exceptional pandemic caused by SARS-CoV-2.
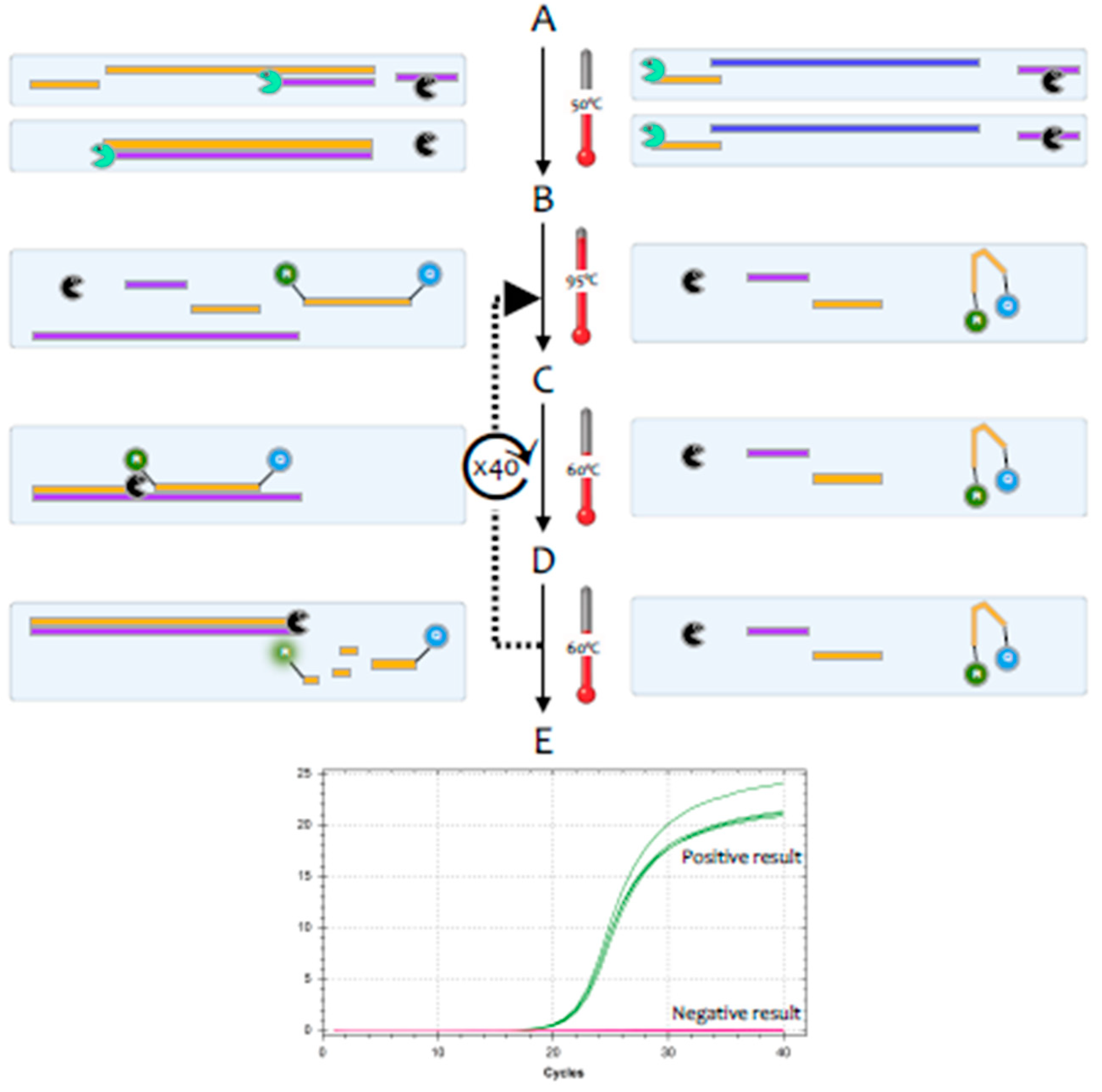
What is sars-cov-2 rt pcr detection. This means that the test can continue to detect fragments of SARS-CoV-2 virus even after youve recovered from COVID-19 and are no longer contagious. RT-PCR is a technology combining RNA reverse transcription RT with polymerase chain amplification PCR of cDNA. Because the PCR test is so sensitive it can detect very small amounts of virus material.
Such assays can alleviate several tests on samples obtained from people who are suspected of having respiratory viral infection which is similar to Covid-19 infection. Although COVID-19 cases due to this variant had clinical biological and radiological findings in line with classical features of COVID-19 B1616 was associated with more severe disease. However understanding whether the RT-PCR test.
MANILA The reverse transcription-polymerase chain reaction RT-PCR test remains the most effective to detect SARS-CoV-2 the virus causing coronavirus disease 2019 Covid-19 Health Undersecretary Maria Rosario Vergeire said Saturday. Beyond 10 days post-symptom onset lower RT or faecal testing may be preferred sampling sites. CDC has developed two laboratory tests that identify SARS-CoV-2 the virus that causes COVID-19.
The low rate of SARS-CoV-2 detection by RT-PCR tests on nasopharyngeal samples within a large cluster of COVID-19 cases led to the identification of a novel variant B1616. RT-PCR misses detection of people with SARS-CoV-2 infection. We read the Article by Lescure and colleagues1 with great interest.
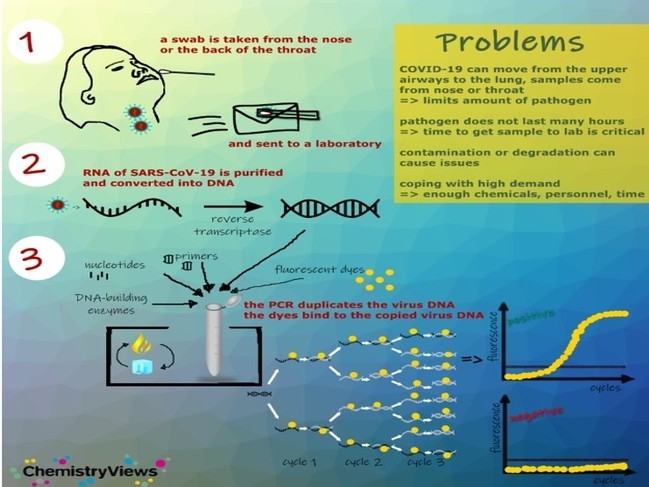
However inaccurate test results for example high false negative rate and some false positive rate were reported in both China and US CDC using the RT-PCR method. To diagnose a SARS-CoV-2 infection now a nasal swab is used to detect the RNA of SARS-CoV-2 virus. Finally using the identified sequences we develop a SARS-CoV-2 specific primer set and test it using a conventional PCR.
Wikramaratna P Paton RS Ghafari M Lourenco J 2020 Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. The included studies are open to substantial. The estimates of posterior median and 95 was available for any of the aforementioned parameters.
The Philippine News Agency is a web-based newswire service of the Philippine government under the supervision of the News and Information. 3 As a fact it has been reported that 3 to 347 of patients with chest computed 1832. These proteins include multiple epitopes.
COVID-19 ddPCR Molecular Test SARS-CoV-2 Droplet Digital PCR ddPCR Kit Our SARS-CoV-2 ddPCR Kit is a partition-based endpoint RT-PCR test for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharyngeal anterior nasal and mid-turbinate nasal swab specimens and nasopharyngeal washaspirate plus nasal aspirate specimens from patients suspected of being infected with COVID-19. Thus non-informative priors beta 1 1 were outcome of K9 dogs and RT-PCR for detection of SARS-CoV-2 used to fit the Bayesian model since no reliable prior information are shown in Table 4. The first test for COVID-19 diagnosis that CDC distributed released in February 2020 is the CDC 2019-Novel Coronavirus 2019-nCoV Real-Time RT-PCR Diagnostic Panel a test that accurately detects SARS-CoV-2 in respiratory specimens.
SARS-CoV-2 nucleic acid detection by RT-PCR is one of the criteria approved by China FDA for diagnosis of COVID-19. This is the COVID-19 PCR test To detect that an infection occurred at some point in the past a serology blood test is done to detect antibodies to SARS. To allow the diagnosis of COVID-19 infections several assays based on the real-time PCR technique have been proposed.
Early sampling minimises false negative diagnoses. Detection of SARS-CoV-2 using real-time polymerase chain reaction in different clinical specimens. The SARS-CoV-2 primers are designed to detect RNA from SARS-CoV-2 genome from the nucleocapsid N gene envelope E gene and ORF1ab region and includes controls for RNase P.
To test for presence of virus in a host human body a sample is. Considerations When Testing SARS-CoV-2 testing may be incorporated as part of a comprehensive approach to reducing transmissionSymptom screening testing and contact tracing are strategies to identify people infected with SARS-CoV-2 so that actions can be taken to slow and stop the spread of the virus. SARS-CoV-2 Mutation Detection Variant-Seq SARS-CoV-2 Kit PKamp VariantDetect SARS-CoV-2 RT-PCR Assay PerkinElmer SARS-CoV-2 Nucleic Acid Detection Kit RUO JANUS G3 Workstations for SARS-CoV-2 Testing Mobile Monitoring for COVID Labs.
After the outbreak of SARS-CoV-2 the Centers for Disease Control. The DxTerity SARS-CoV-2 RT-PCR CE Test is an end point reverse transcription polymerase chain reaction RT-PCR test followed by detection with capillary electrophoresis from Saliva Samples. Moreover real-time RT-PCR has adequate sensitivity to help us much for diagnosing early infection.
It is a commonly used molecular diagnostic tool to detect coronavirus including SARS-CoV and MERS-CoV in human clinical specimens. SARS-CoV-2 2019-nCoV antigen detection. Based on the principle of specific binding of antigens to antibodies the presence of antigens can be detected directly by antibodies thereby directly.
The novel coronavirus gene encodes multiple structural proteins such as N protein E protein and S protein. Nevertheless according to recent evidence detection rate of SARS-CoV-2 RNA on the grounds of RT-PCR performed on both NPS and OPS would be lower than expected. Apart from the possible delay in getting treatment itself there are also questions about whether the new variants of the SARS-CoV-2 virus found in.
RT-PCR Assay for CoV Detection. As such real-time reverse transcriptase-PCR RT-PCR is of great interest today for the detection of SARS-CoV-2 due to its benefits as a specific and simple qualitative assay 13. Inaccurate results are caused by inadequate detection sensitivity of RT-PCR low viral load in some.
Findings A first validation performed on 583 samples from the NGDC repository and 20604 from the NCBI repository show. A critical review Allergol Immunopathol Madr. COVID SARS Coronavirus 2 PCR Detect V Overview Useful For Detection of coronavirus disease 2019 COVID-19 illness due to severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Recommended only for patients who meet current.
A multi-analyte reverse transcription-polymerase chain reaction RT-PCR test such as PKamp Respiratory SARS-CoV-2 RT-PCR Panel 1 permits labs to preserve valuable resources. COVID-19 virus involves the following steps.

Rapid Detection Of Sars Cov 2 Replicating Or Non Replicating Using Rt Pcr International Journal Of Infectious Diseases
Molecular Mechanisms And Methods Used To Detect Covid 19 Infection News Cordis European Commission

Powerful Versatile Sars Cov 2 Detection
.png)