To perform the SARS-CoV-2 RNA Qualitative Real-Time RT-PCR test SARS-CoV-2 nucleic acid is first extracted isolated and purified from upper and lower respiratory specimens such as of. The test code is 39448.
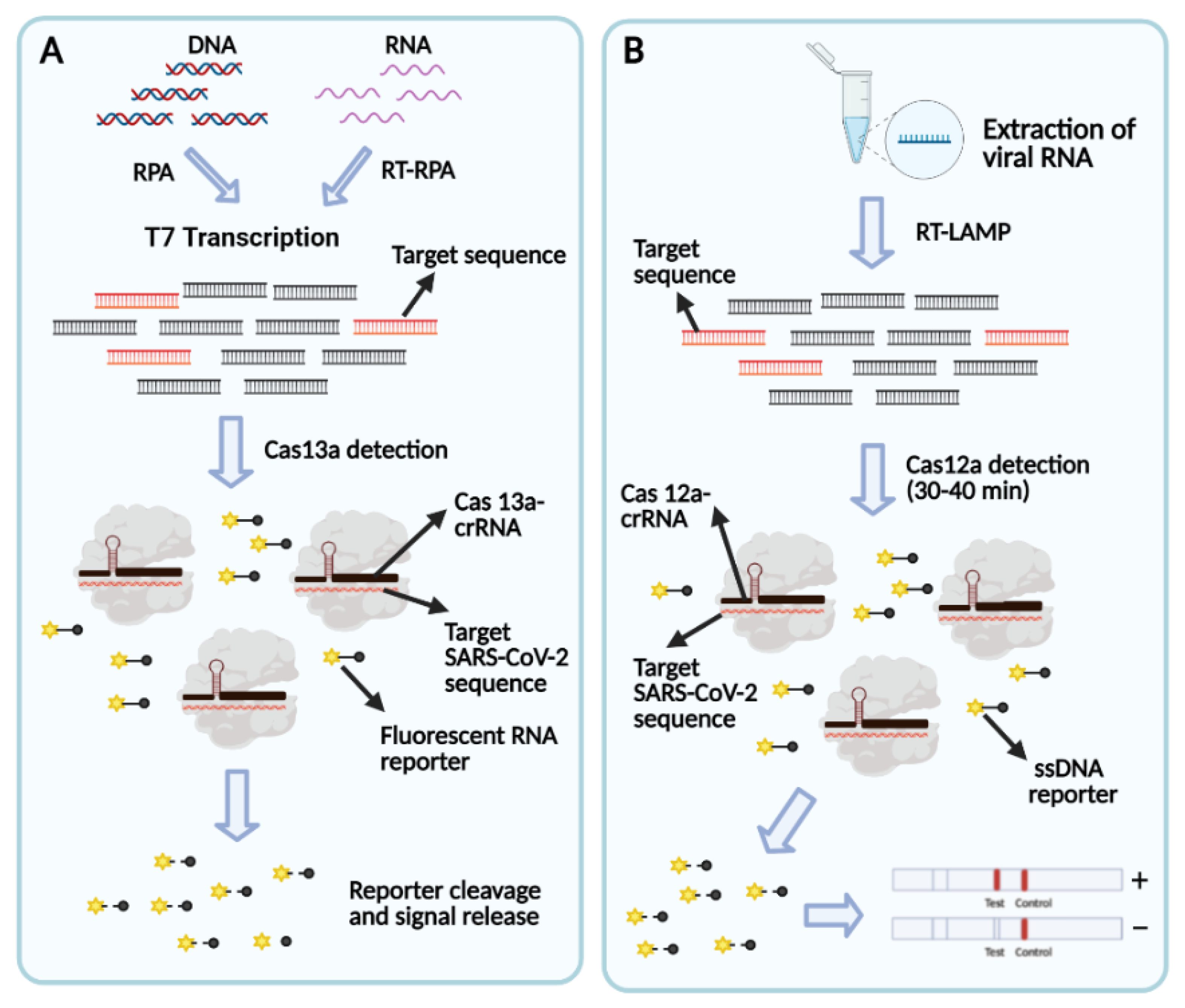
Bioengineering Free Full Text Covid 19 Diagnostic Strategies Part I Nucleic Acid Based Technologies Html
Amplification of both targets results in a presumptive positive detectable test result while amplification of one of two targets results in an inconclusive result and amplification of neither target results a negative non-detectable test result.
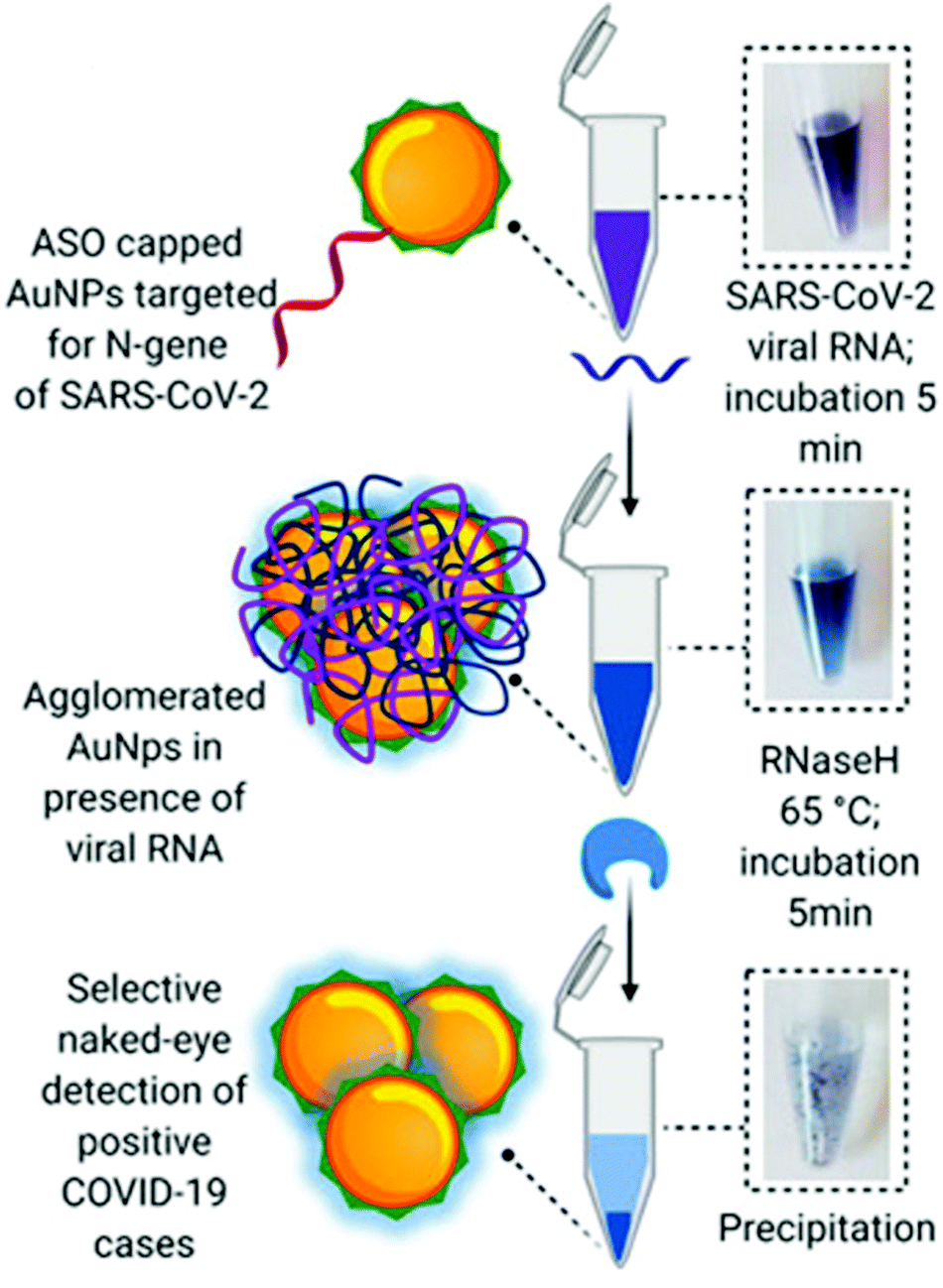
What is sars cov 2 rna tma. SARS-CoV-2 is closely related to the original SARS-CoV. March 9 2020 You are being given this Fact Sheet because your samples were tested for the Coronavirus Disease 2019 COVID9 -1 using the Quest Diagnostic SARS-CoV-2. TMA provides an effective highly sensitive means of detection of SARS-CoV-2 in nasopharyngeal specimens.
COVOO SARS Coronavirus 2 RNA PCR V Overview Useful For Diagnosis of coronavirus disease 2019 COVID-19 illness due to severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Recommended only for patients who meet current. The UW SARS-CoV-2 Real-time RT-PCR assay targets two distinct regions within the N gene of SARS-CoV-2 the causative agent for COVID-19. Poplar SARS-CoV-2 TMA Pooling assay EUA Summary 2 Testing is limited to Poplar Healthcare located at 3495 Hack Cross Rd Memphis TN 38125 that is certified under the Clinical Laboratory.
This work helps support the HHS COVID-19 Pandemic Response Laboratory Data Reporting. Both assays enable labs to run over 1000 tests in 24 hours and attain first results in 35 hours or less. The emergence of SARS-CoV-2 was unforeseen and the worldwide outbreak of COVID-19 has already impacted thousands.
Molecular Assays for Detection of Viral Nucleic Acids SARS-CoV-2 is a single-stranded positive-sense RNA virus and since its entire genetic sequence was uploaded to the Global Initiative on Sharing All Influenza Data GISAID platform on January 10 2020. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus in subgenus Sarbecovirus lineage B together with two bat-derived strains. Both PCR and TMA based assays are very sensitive at detecting the virus especially within the first week after symptoms develop.
Although other diagnostic methods have been introduced detection of viral genes on oro- and nasopharyngeal swabs by reverse-transcription real time. This test is intended to be performed on. SARS-CoV-2 antigen tests detect a part of the virus called viral proteins which make up the viruss.
Syndrome coronavirus 2 SARS-CoV-2 is the causative agent of Coronavirus Disease 2019 COVID-19. In response to the desperate public need for rapid high-volume testing we have developed the Panther Fusion SARS-CoV-2 assay which enables labs to run up to 1150 tests in 24 hours and attain a time-to-first result in 24 hours. Higher analytical sensitivity may explain TMAs ability to ascertain for the presence of SARS-CoV-2 genome in human specimens deemed inconclusive by real-time PCR.
Nucleic Acid Amplification Tests NAATs A Nucleic Acid Amplification Test or NAAT is a type of viral diagnostic test for SARS-CoV-2 the virus that causes COVID-19. Reverse transcriptase PCR rt-PCR. Specimens are to be collected by hospitals physician offices and.
The genomic material for the SARS-CoV-2 virus is ribonucleic acid RNA which remains in the body while the virus is still replicating and reproducing. New insights on antibody testing and RNA testing. Diagnostic tests look for evidence of this replication processthat more viruses are being madeto diagnose an active infection of COVID-19.
Detect the part of the SARS-CoV-2 virus called viral RNA nucleic acid which is the viruss genetic material. NAATs detect genetic material nucleic acids. Many hypotheses involving recombination convergence and adaptation have been put forward to suggest a probable evolutionary pathway for SARS-CoV-2 but none is supported by direct evidence.
Molecular tests are different from antigen tests. The Regenstrief LOINC team has been working closely with APHL CDC FDA labs IVD manufacturers and other stakeholders on terminology specifically related to SARS-CoV-2COVID-19. The Panther Fusion SARS-CoV-2 assay is a real-time PCR test and the Aptima SARS-CoV-2 assay utilizes our proprietary TMA technology.
All test specific information can be found in the test directory. The test name is SARS-CoV-2 RNA COVID-19 Qualitative NAAT. Two types of tests are used to track SARS-CoV-2.
Since its emergence at the end of 2019 in Wuhan City China the virus has caused an ongoing global outbreak of COVID-19 and a major global public health emergency. Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 is the etiologic agent of the COVID-19 pandemic. They are closely related to both SARS-CoV-2 and RaTG13 but apparently they are unlikely the immediate ancestor of SARS-CoV-2 in view of the sequence divergence over the whole genome.
Considerations When Testing SARS-CoV-2 testing may be incorporated as part of a comprehensive approach to reducing transmissionSymptom screening testing and contact tracing are strategies to identify people infected with SARS-CoV-2 so that actions can be taken to slow and stop the spread of the virus. 66 It is thought to have an animal zoonotic origin. Both assays are intended for the qualitative detection of RNA from SARS-CoV-2 isolated and.
CARES Act Section 18115 requirements that were published on June 4 2020. To diagnose a SARS-CoV-2 infection now a nasal swab is used to detect the RNA of SARS-CoV-2 virus. This is the COVID-19 PCR test To detect that an infection occurred at some point in the past a serology blood test is done to detect antibodies to SARS.
Nucleic Acid Amplification Testing for Individuals With a Previous Positive SARS-CoV-2 Test Result NAATs can detect SARS-CoV-2 RNA in specimens obtained weeks to months after the onset of COVID-19 symptoms. SARS-CoV-2 RNA COVID-19 Qualitative NAAT - The SARS-CoV-2 RNA COVID-19 Nucleic-acid Amplification Test NAAT is a qualitative multi-target molecular diagnostics test that aids in the detection of COVID-19. NAATs for SARS-CoV-2 specifically identify the RNA ribonucleic acid sequences that comprise the genetic material of the virus.
12 Due to the RNA-based nature of the SARS-CoV-2 genome the mutation rate is anticipated to be high. What facilities can collect specimens. Interpreting SARS-CoV-2 RNA Qualitative Real-Time RT-PCR Test Results Updated.
1314 However the likelihood of recovering.

Absence Of Sars Cov 2 Covid 19 Virus Within The Ivf Laboratory Using Strict Patient Screening And Safety Criteria Reproductive Biomedicine Online
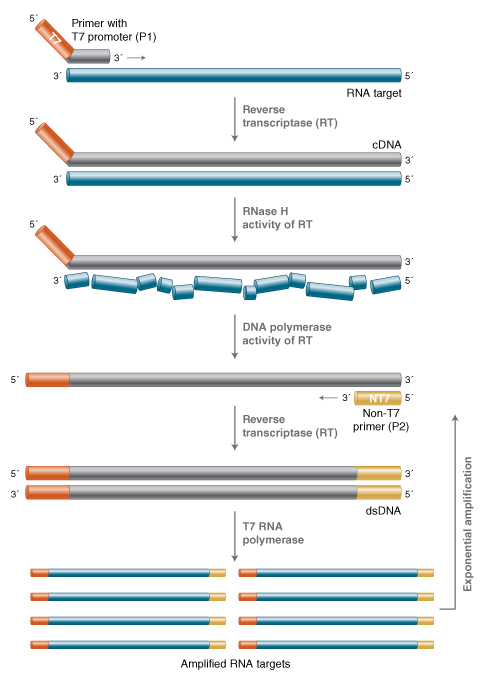
Transcription Mediated And Nasba Amplification Neb
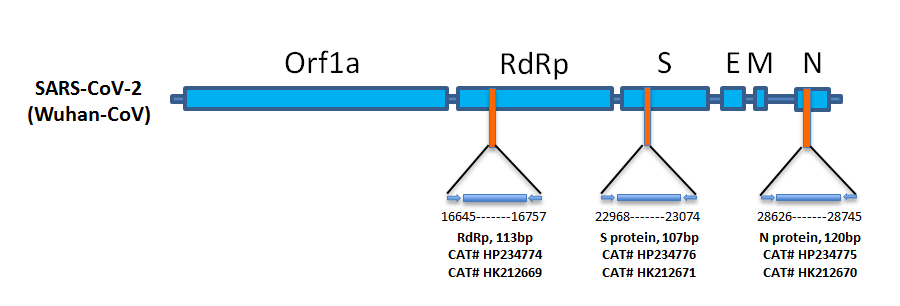
Sars Cov 2 Covid 19 N Protein Gene Qpcr Template Standard Hk212670 Origene
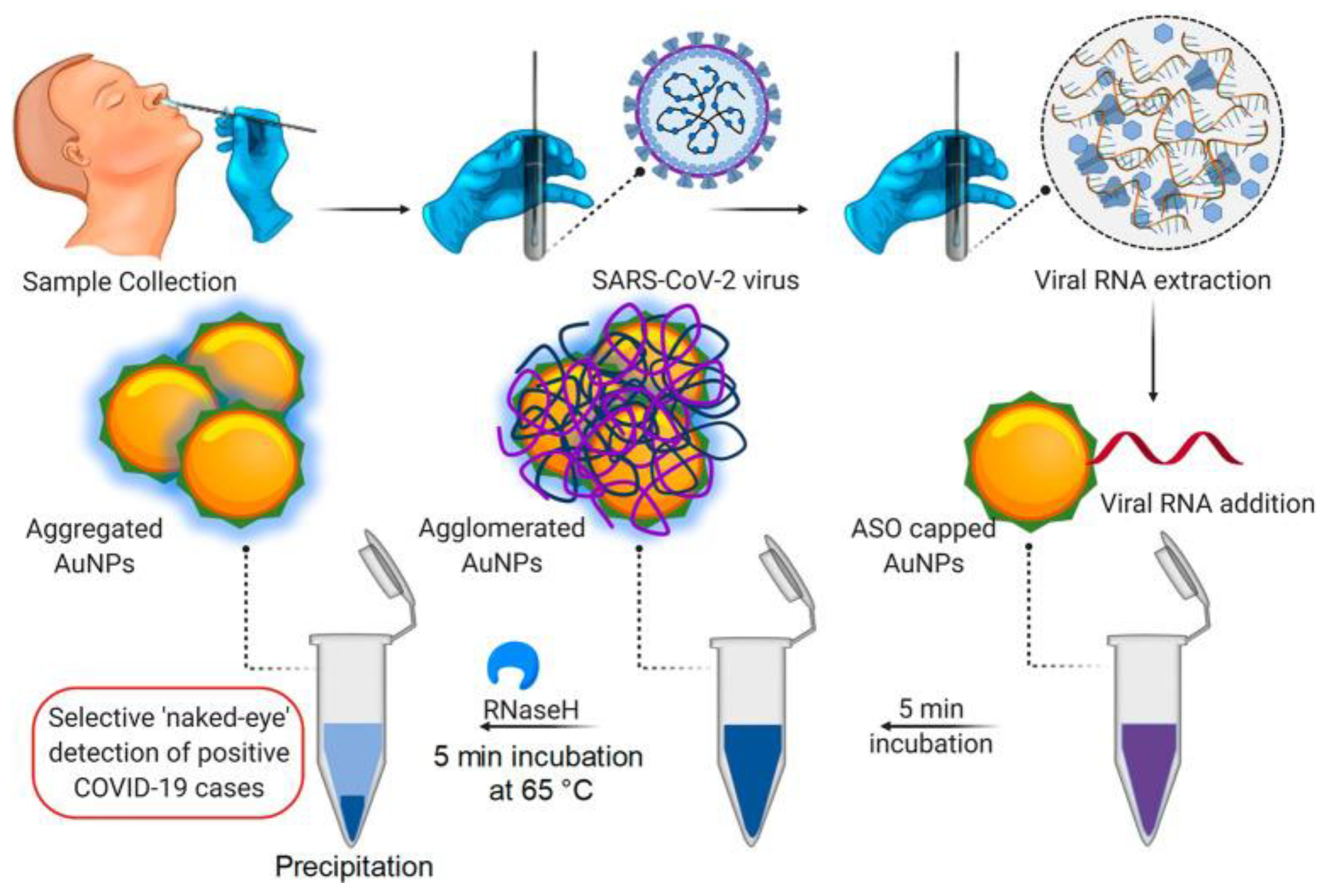
Microorganisms Free Full Text A Comprehensive Review Of Detection Methods For Sars Cov 2 Html